Mass spectrometry is a technique used to analyze the chemical composition of a sample. It uses ionizing radiation to separate the molecules of the sample into their constituent ions, which are then separated according to their mass-to-charge ratio. Mass spectrometry has been used since the early 19th century, but the principles behind it have been known about 100 year earlier.
English chemist John Dalton proposed that molecules could be broken down into smaller particles of different masses, thus allowing them to be analyzed according to their mass. This concept was further developed by French chemist Joseph Louis Gay-Lussac in 1808 when he proposed the law of multiple proportions, which stated that when two elements combine to form a compound, the ratio of the masses of the elements will be a whole number. This law is the basis for the modern idea of isotopes and the development of mass spectrometry. In 1856, German chemist Robert Bunsen and British chemist Sir William Crookes developed the first mass spectrograph (still not spectrometer!), which allowed them to measure the masses of molecules. This device was further refined and improved upon in the early 20th century by pioneers such as Sir J.J. Thomson and Sir Ernest Rutherford, who are credited with discovering the first isotopes and the atomic mass unit, respectively.
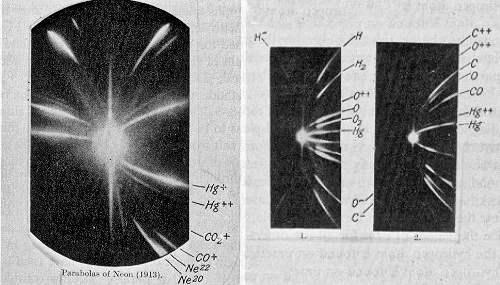
One of the first results using mass spectrometry
An improved version of Thomson’s mass spectrograph was used in 1919 by Francis William Aston, who used mutually perpendicular fields instead of electric and magnetic fields parallel to each other. The electric field splits the ion beam according to the mass to charge ratio, but also according to different velocities. By selecting appropriate field strengths, one can cause the magnetic field to direct all particles of different velocities to one specific point in space, while leaving particle beams of different m/q separated. In this way, the spectrograph has a higher transmission for ions so that a higher separation capacity is achieved. Aston’s invention of the mass spectrometer also enabled the discovery of isotopes, which he used to calculate the atomic weights of many elements. His research also helped to advance the field of nuclear physics and nuclear chemistry.
In the first half of the 20th century, mass spectrometry was in its infancy. Early mass spectrometers used vacuum tubes and magnets and were very inefficient. However, advances in the technology allowed for more precise measurements to be taken and the development of modern mass spectrometers. By the mid-20th century, mass spectrometry had become an important tool for chemists and physicists to study the structure and behavior of molecules.
Early mass spectrometers used vacuum tubes and magnets and were very inefficient. However, advances in the technology allowed for more precise measurements to be taken and the development of modern mass spectrometers. By the mid-20th century, mass spectrometry had become an important tool for chemists and physicists to study the structure and behavior of molecules.
Next decades of 20th century, mass spectrometry began to be used more widely in the analysis of organic compounds. Scientists developed techniques such as electron impact mass spectrometry and chemical ionization mass spectrometry to better understand the structure of molecules.
In the second half of the 20th century, advances in technology allowed scientists to explore the field further. Instruments such as time-of-flight mass spectrometers, Fourier transform ion cyclotron resonance mass spectrometers, and quadrupole mass spectrometers allowed for greater precision in the analysis of samples. Currently, coupled techniques are used, such as mass spectrometry with gas chromatography, which has become a standard in the precise identification of molecules