Pure ethanol freezes at about -114 °C. However, by adding more and more water to this alcohol, the freezing point of such a solution begins to approach 0 °C. For example, vodka, a 40% solution of ethanol and water, freezes at -23 °C. Decent freezers are already able to reach such temperatures. Knowing at what temperature a given alcohol solution freezes can help you check the manufacturer for “baptism” by adding too much water, thus reducing its “percentage”. See below a graph of how the freezing point corresponds to the ethanol content of the aqueous solution.
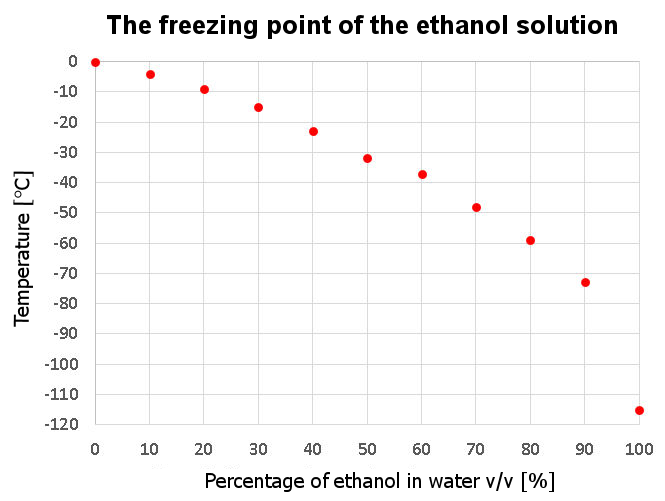
Graph of the freezing of an ethanol solution depending on the percentage of alcohol content
Based on the graph above, we can estimate that a typical beer that contains 5% ethanol will freeze at a temperature of about -2 °C. Lighter wine, on the other hand, which has about 10% alcohol in it, will freeze at -4 °C. A stronger version of the wine, which will start to turn into a solid at around -9 °C, closer to 20%. The vodka mentioned earlier freezes at -23 °C. A typical absinthe with a “power of” 70% will only turn ice at -48°C. Now, when you know a little bit more about ethanol freezing point, you will not be so easily fooled 😉






