Water, although it is a very simple molecule, because it consists of only 3 atoms – one oxygen and two hydrogen – is the foundation of life on Earth, and probably in the universe at all (if we find one). It is not without reason that when observing distant solar systems, astronomers look for planets that are in the so-called habitable zone, i.e. the habitable zone enabling, among others, the occurrence of liquid water. But let’s go back to Earth and look at water from the side of its properties important for life …
Water as a chemical molecule
When we start our discussion of water, let us begin by listing the basic properties related to its structure. As mentioned, the water molecule is made up of two hydrogens and oxygen, which is simply H2O. It seems uncomplicated and as simple as it gets. However, this simplicity has complex consequences. The oxygen atom is connected to both hydrogens by a pair of covalent bonds. These bonds are polar, which means that in such a case there is an uneven distribution of partial electric charges. It is expertly said that such a molecule has a non-zero electric dipole moment. In the case of water, the negative charge is shifted towards the oxygen atom and the positive charge is located near the hydrogen nuclei. The structure of the water molecule and the distribution of the electric charge is presented in the figure below:
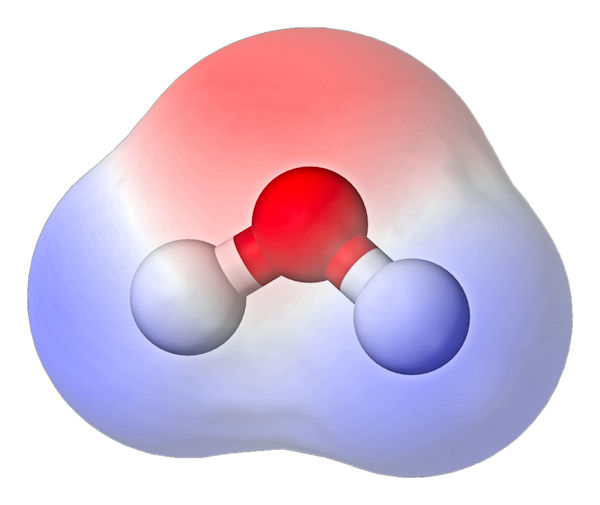
Water molecule (red ball – oxygen, white balls – hydrogen) and electric charge distribution (red cloud – negative charge, blue – positive charge)
It is the polarity that causes H2O molecules to easily hydrogen bond with other molecules. When water molecules are bound together, one molecule of H2O can form a hydrogen bond with up to 4 other water molecules (which can be observed during ice formation).
Water as a solvent
The polar nature of water is also manifested in the fact that many substances can be dissolved in it. This situation occurs when the hydrogen bonds produced by H2O replace weaker bonds in other chemicals. As a result, the aqueous solution may contain many substances necessary for the cell’s biochemistry. There is a reason why such cytoplasm is mostly water. It is worth mentioning here about lipids that have a non-polar part, water-insoluble (hydrophobic), which can form closed membranes, separating the areas with water from the environment.
Surface tension
Another important property of water is its surface tension. It is a physical quantity representing the energy per unit area of water resulting from the attraction forces between the molecules of the liquid. In short, it is a measure of the force that must be applied to increase the surface of the liquid by a given value, that is, to move the particles apart acting against the forces by which they attract each other. For water with a temperature of 18 °C it is about 72.4 · 10−3 N / m.
It is the surface tension phenomena that are responsible for the shape of the droplets and allow certain materials to remain on the surface of the water as it behaves like an elastic film. Again, hydrogen bonds, which are not the weakest, are responsible for these attractive forces between molecules. One of the important consequences of this phenomenon is the fact that the water in the thin capillary can move “upwards”, and more precisely against the gravitational pull. The capillary phenomenon also allows the water column to be maintained, which is crucial for the transport of this liquid, e.g. in plants, as exemplified by trees reaching over 100 meters (the record belongs to the evergreen redwood called Hyperion, measuring 115.61 meters!)
Water as a heat store
The thermal properties of water, i.e. how it can store and transfer heat, are other parameters important for organisms. Heat is one form of energy transfer after all, and without energy there is no life. Water, in turn, has it that on the one hand you need to put a lot of energy (specific heat: 4187 J / kg K) to raise the temperature by another degree (increase the average kinetic energy of molecules), and also to “disconnect” from each other molecules, breaking the hydrogen bonds between the H2O molecules (heat of vaporization: 2257 kJ / kg). It is of great importance for living organisms in thermoregulation processes – in the case of plants, the evaporating water on the leaf surface protects against excessive absorption of solar energy, and in the case of animals of this type, the process of sweating plays a role.
Water density
Water increases in volume when it freezes, which is perhaps quite counter-intuitive. All this is due to the formation of a crystal lattice along with the temperature drop, in which there is a lot of free space between the permanently set water particles. This leads to a situation where ice is less dense than liquid water and can therefore float on its surface. This causes water reservoirs to freeze “from above”, not from the bottom, so that aquatic organisms can survive under the ice that is pushed to the surface of the reservoir.
The highest density (approx. 999.973 kg/m3) is achieved by water at 4 °C. At a temperature of 0 °C, the density of water is about 999.841 kg/m3, and for a temperature of 25 °C, the density will be about 997.044 kg/m3.